A multilayered analysis of progeroid syndrome to elucidate the pathophysiology of aging and disease

-
- Principal Investigator
Associate Professor / Yoshiro MAEZAWA
- Affiliation
Graduate School of Medicine, Chiba University
Researchmap
ORCID ID
- Principal Investigator
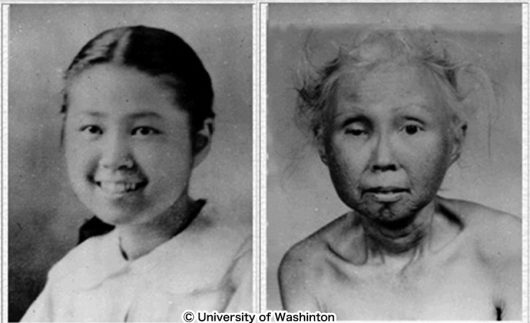
In this study, we focus on Werner's syndrome, a representative hereditary progeroid syndrome associated with high rates of atherosclerosis, diabetes and cancer. Werner syndrome is a rare intractable disease caused by mutations in the WRN gene, a DNA helicase. The patients with Werner syndrome show early onset of bilateral cataract, skin atrophy and intractable ulcers, diabetes, atherosclerosis, fatty liver and malignant tumors. The symptoms of Werner syndrome is shares many aspects with normal aging, and it is considered a model disease of human aging. Through this analysis, we will elucidate the pathophysiology of Werner syndrome as well as general human aging. However, the knockout (KO) mice of the WRN gene do not show symptoms of early aging, which has been a major barrier for study progression.
We have developed the world's first primate model of Werner's syndrome, the WRN KO marmoset. Because the telomere length of the marmoset is similar to that of human, we expect that WRN KO marmoset will show premature aging phenotype. We also will elucidate the mechanism of premature aging by analysis of fibroblasts and iPS cells (WS-specific iPS cells) established from patients with Werner syndrome. We also established differentiation method into mesenchymal stem cells, adipocytes, vascular wall cells and macrophages from WS-specific iPS cells. We will analyze and clarify the mechanism of immune system and pathogenesis of Tumorigenesis in the premature aging condition. Until now we observed that Werner syndrome cells show early cellular senescence, elevated expressions of inflammatory cytokines called SASP (Senescence associated secretary phenotype), altered differentiation status and dysregulation of intercellular communication.
By analyzing these prematurely senescent cells using various cutting-edge methods including proteomic and metabolomic techniques, we will elucidate the molecular mechanisms of senescence and identify genes that suppress or promote senescence. Furthermore, we will identify the therapeutic targets that regulate senescence.
This research will lead to the development of innovative new treatments for this intractable disease. In addition, by clarifying the mechanisms of aging, we hope to elucidate the background pathology of aging-related diseases such as cancer, atherosclerosis, diabetes and osteoporosis, which will lead to breakthroughs in the treatment of these diseases.